FDA Advisory Committee Recommends Approval of CAR-T Cell Therapy and Two New Biosimilars | Biosimilars Law Bulletin
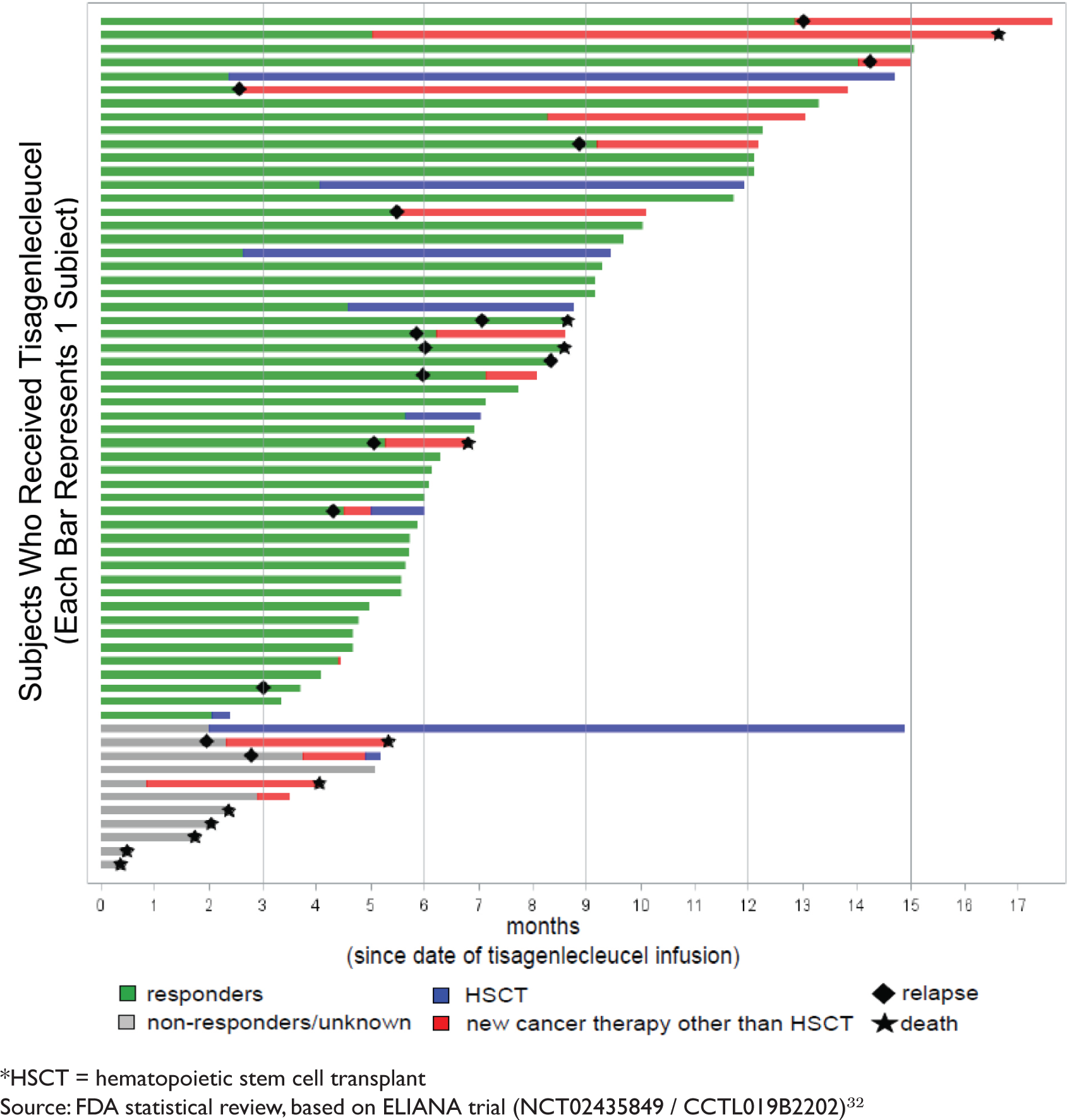
ONCOLOGIC DRUGS ADVISORY COMMITTEE BRIEFING DOCUMENT Tisagenlecleucel (CTL019) for the TREATMENT OF PEDIATRIC AND YOUNG ADULT PA
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
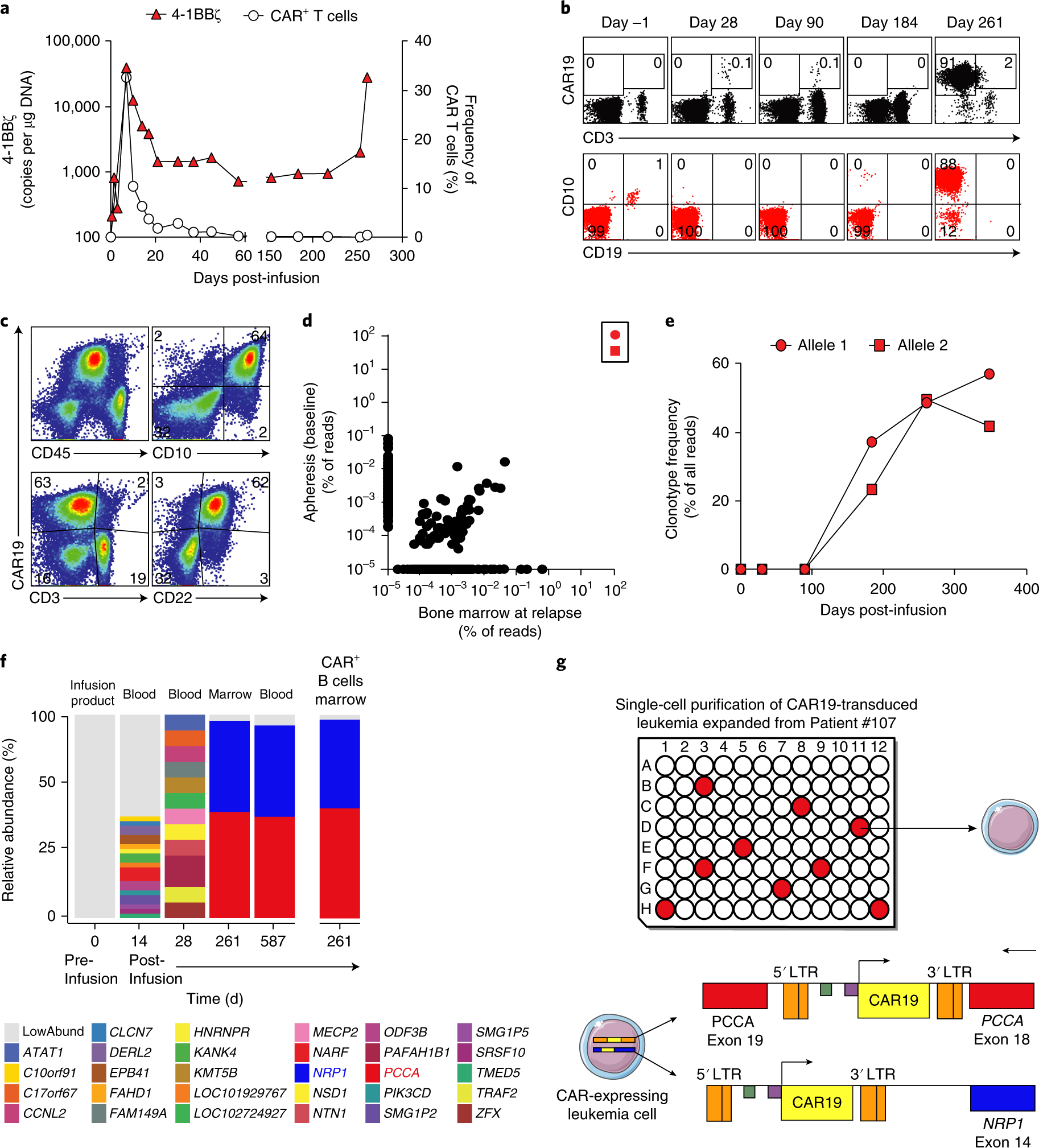
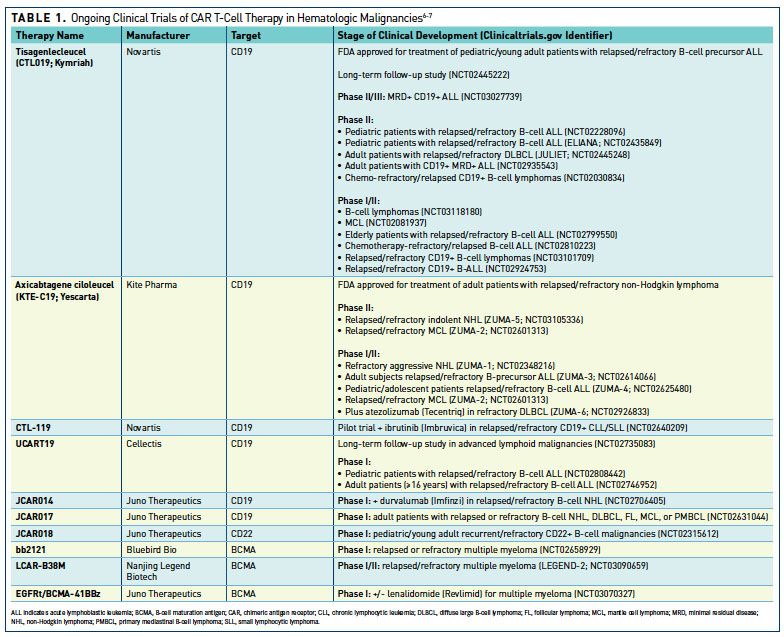
Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell | Nature Medicine
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
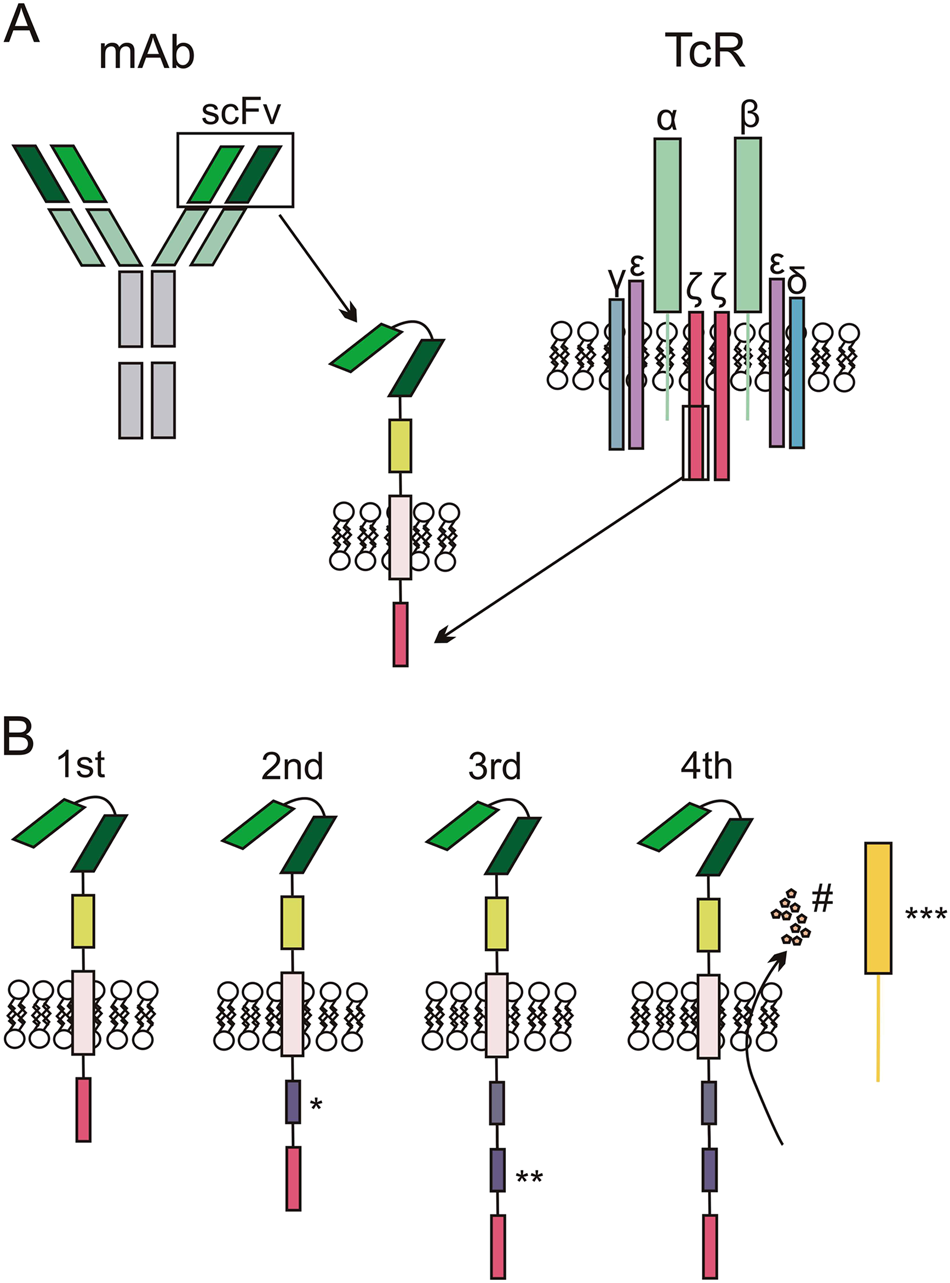
The biological basis and clinical symptoms of CAR-T therapy-associated toxicites | Cell Death & Disease

Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma | Nature Communications
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar